2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be? Boyle's law and charles's law can be combined to form the ideal gas law, a single generalization of the behavior of gases known as an equation of state. Gas laws, laws that relate the pressure, volume, and temperature of a gas. Combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? These include the buffers that you use in lab classes.
 Nfschools Net from The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. Propane (c 3 h 8) reacts with oxygen in a [filename: You need to comprehend how to project cash flow. Newton's laws of motion review navigate to: A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. These include the buffers that you use in lab classes. Limiting reactant and percent yield practice worksheet answers. Limiting reactant date _____ 1.
Nfschools Net from The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. Propane (c 3 h 8) reacts with oxygen in a [filename: You need to comprehend how to project cash flow. Newton's laws of motion review navigate to: A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. These include the buffers that you use in lab classes. Limiting reactant and percent yield practice worksheet answers. Limiting reactant date _____ 1.
Newton's laws of motion review navigate to:
2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be? Limiting reactant date _____ 1. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? If the temperature where the balloon is released is 20 0 c, what will happen These include the buffers that you use in lab classes. Mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? You need to comprehend how to project cash flow. This interest can be harnessed to teach an exciting lesson on gas laws. Boyle's law and charles's law can be combined to form the ideal gas law, a single generalization of the behavior of gases known as an equation of state. Students are often fascinated by extreme sports such as scuba diving. 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. Learn more about gas laws in this article. Combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm?
2) if i initially have a gas with a pressure of 84 kpa and a … 2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be? Propane (c 3 h 8) reacts with oxygen in a [filename: Each of these laws can be derived from this law. The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems.
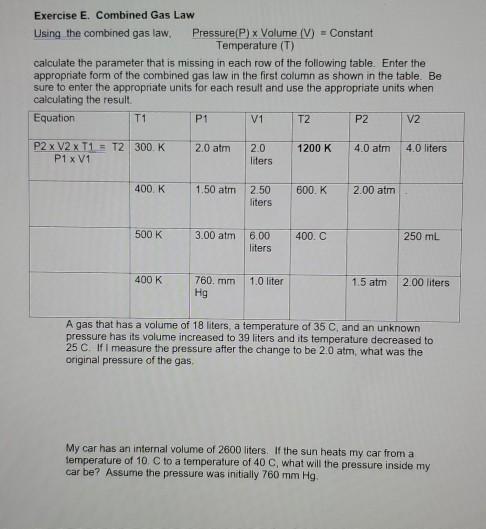 Solved Exercise E Combined Gas Law Using The Combined Gas Chegg Com from media.cheggcdn.com Gas laws, laws that relate the pressure, volume, and temperature of a gas. Limiting reactant date _____ 1. Newton's laws of motion review navigate to: If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Propane (c 3 h 8) reacts with oxygen in a [filename: These include the buffers that you use in lab classes. The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Solved Exercise E Combined Gas Law Using The Combined Gas Chegg Com from media.cheggcdn.com Gas laws, laws that relate the pressure, volume, and temperature of a gas. Limiting reactant date _____ 1. Newton's laws of motion review navigate to: If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Propane (c 3 h 8) reacts with oxygen in a [filename: These include the buffers that you use in lab classes. The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm?
Limiting reactant date _____ 1. You need to comprehend how to project cash flow. The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. The correct answer is given in parentheses at the end of the problem. Students are often fascinated by extreme sports such as scuba diving. 3) 0a balloon is filled with 35.0 l of helium in the morning when the temperature is 20.0 c. Propane (c 3 h 8) reacts with oxygen in a [filename: If the temperature where the balloon is released is 20 0 c, what will happen A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. A as ple contained in a cylinder equipped with a moveable piston occupie 00.0 at a pressure These include the buffers that you use in lab classes. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? Gas laws, laws that relate the pressure, volume, and temperature of a gas.
If the temperature where the balloon is released is 20 0 c, what will happen The correct answer is given in parentheses at the end of the problem. These include the buffers that you use in lab classes. Limiting reactant date _____ 1. 2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be?
 Practice Charles S Gas Law Worksheet 1 0 Answer Key By The Chem Teacher from ecdn.teacherspayteachers.com Newton's laws of motion review navigate to: 2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be? If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. 2) if i initially have a gas with a pressure of 84 kpa and a … A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. You need to comprehend how to project cash flow. The correct answer is given in parentheses at the end of the problem. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Practice Charles S Gas Law Worksheet 1 0 Answer Key By The Chem Teacher from ecdn.teacherspayteachers.com Newton's laws of motion review navigate to: 2) if 5.0 moles of o 2 and 3.0 moles of n 2 0are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the resulting mixture of gases be? If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. 2) if i initially have a gas with a pressure of 84 kpa and a … A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. You need to comprehend how to project cash flow. The correct answer is given in parentheses at the end of the problem. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature?
Newton's laws of motion review navigate to:
Combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? The ideal and combined gas laws pv = nrt or p 1v 1 = p 2v 2 t 1 t 2 use your knowledge of the ideal and combined gas laws to solve the following problems. Each of these laws can be derived from this law. Propane (c 3 h 8) reacts with oxygen in a [filename: The correct answer is given in parentheses at the end of the problem. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? What is the new volume? 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. A as ple contained in a cylinder equipped with a moveable piston occupie 00.0 at a pressure 3) 0a balloon is filled with 35.0 l of helium in the morning when the temperature is 20.0 c. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. 2) if i initially have a gas with a pressure of 84 kpa and a … This interest can be harnessed to teach an exciting lesson on gas laws.
Gas Laws Worksheet Answers / Article Gas Laws Scuba Diving X10hosting New Account Pages 1 4 Flip Pdf Download Fliphtml5 :. Propane (c 3 h 8) reacts with oxygen in a [filename: Limiting reactant date _____ 1. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? What is the new volume? Gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle's law problems: